 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Alesion, Elestat, Purivist, Relestat |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604011 |
| Pregnancy category |
|
| Routes of administration | Eye drops |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | 64% |
| Elimination half-life | 12 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.120.187 |
| Chemical and physical data | |
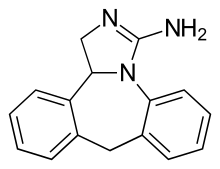
| Formula | C16H15N3 |
| Molar mass | 249.317 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Epinastine (brand names Alesion, Elestat, Purivist, Relestat) is a second-generation antihistamine and mast cell stabilizer that is used in eye drops to treat allergic conjunctivitis. It is produced by Allergan and marketed by Inspire in the United States.[1] It is highly selective for the H1 receptor and does not cross the blood-brain-barrier.[2]
It was patented in 1980 and came into medical use in 1994.[3]
References
- ↑ Pradhan S, Abhishek K, Mah F (September 2009). "Epinastine: topical ophthalmic second generation antihistamine without significant systemic side effects". Expert Opinion on Drug Metabolism & Toxicology. 5 (9): 1135–1140. doi:10.1517/17425250903117284. PMID 19630694. S2CID 207490591.
- ↑ Walther G, Daniel H, Bechtel WD, Brandt K (April 1990). "New tetracyclic guanidine derivatives with H1-antihistaminic properties. Chemistry of epinastine". Arzneimittel-Forschung. 40 (4): 440–446. PMID 1972625.
- ↑ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 549. ISBN 9783527607495.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.