 | |
| Clinical data | |
|---|---|
| Trade names | Youxitai |
| Other names | MRX-I |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
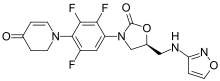
| Formula | C18H15F3N4O4 |
| Molar mass | 408.337 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Contezolid (trade name Youxitai) is an antibiotic of the oxazolidinone class.[1][2] It is effective against Staphylococcus aureus, methicillin-resistant Staphylococcus aureus (MRSA), Streptococcus pyogenes, Streptococcus agalactiae, and other bacteria.[3]
In 2021, it was approved by the National Medical Products Administration of China for the treatment of complicated skin and soft tissue infections (cSSTI).[3][4]
A prodrug of contezolid, contezolid acefosamil, which is formulated for IV administration[5] is in Phase III clinical trials for diabetic foot infection.[6]
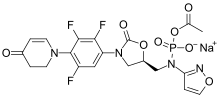 Chemical structure of contezolid acefosamil
Chemical structure of contezolid acefosamil
References
- ↑ Gordeev MF, Yuan ZY (June 2014). "New Potent Antibacterial Oxazolidinone (MRX-I) with an Improved Class Safety Profile". Journal of Medicinal Chemistry. 57 (11): 4487–4497. doi:10.1021/jm401931e. PMID 24694071.
- ↑ Zhao X, Huang H, Yuan H, Yuan Z, Zhang Y (May 2022). "A Phase III multicentre, randomized, double-blind trial to evaluate the efficacy and safety of oral contezolid versus linezolid in adults with complicated skin and soft tissue infections". The Journal of Antimicrobial Chemotherapy. 77 (6): 1762–1769. doi:10.1093/jac/dkac073. PMID 35265985.
- 1 2 Hoy SM (September 2021). "Contezolid: First Approval". Drugs. 81 (13): 1587–1591. doi:10.1007/s40265-021-01576-0. PMC 8536612. PMID 34365606.
- ↑ Mak E (3 June 2021). "Micurx wins China approval for antibacterial contezolid". BioWorld.
- ↑ Liu J, Wang W, Wang C, Zhang L, Zhang X, Liu S, et al. (July 2022). "Discovery of Antibacterial Contezolid Acefosamil: Innovative O-Acyl Phosphoramidate Prodrug for IV and Oral Therapies". ACS Medicinal Chemistry Letters. 13 (7): 1030–1035. doi:10.1021/acsmedchemlett.2c00191. PMC 9290071. PMID 35859881.
- ↑ "Contezolid acefosamil by MicuRx Pharmaceuticals for Diabetic Foot Infection (DFI): Likelihood of Approval". GlobalData. 31 May 2023 – via Pharmaceutical Technology.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.