You may remember my question here, part of which assumed that all humans would die in one way very quickly, with minimal destruction to the planet.
This question will ask about the way humans would die:
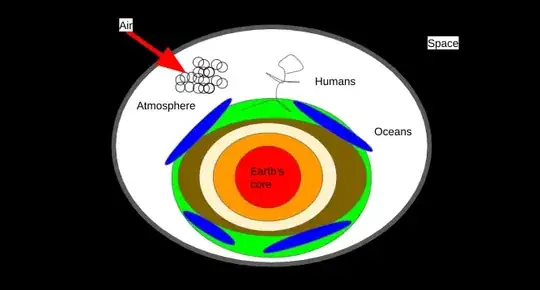
We all know about water's phase changes.
Let's say that the core of the Earth overheated, sending heat waves upward to the surface. The heat vaporizes most of the water, sending gas (steam) into the atmosphere. The gas pushes out most of the air into space and only steam remains. This effectively wipes out the human race in hours.
After hundreds of years, the steam contracts back onto the surface of the planet after Earth's core cools down due to human inactivity¹.
Is this a plausible theory? Could it actually work if Earth overheated?
Some quick diagrams made by me:
Before overheating of Earth:
 Overheating:
Overheating:
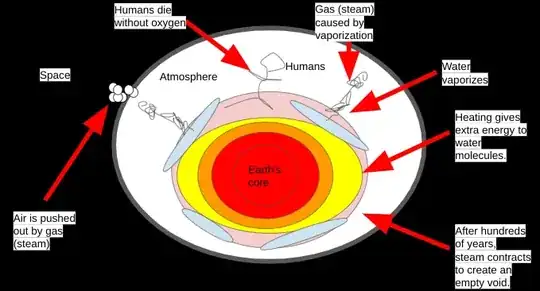
¹As pointed out in comments, humans probably couldn't affect the Earth's core, but just assume it did for the moment.
I am relying on little knowledge in the scientific field so just tell me if I am being an idiot and my theory is bonkers.
- Place a small amount of water in an Erlenmeyer Flask.
- Place an alcohol burner under a mesh stand and have the Erlenmeyer Flask on top.
- Light your burner.
- Heat your Erlenmeyer flask until you have a steady stream of steam.
- Put a balloon on top of your flask.
- Allow cooling for 3 minutes.
– user11111111111 Feb 01 '21 at 21:33