Modelling of atmospheric loss is a complicated thing. There are a bunch of ways in which a planet can lose its atmosphere into space, but probably the most interesting one here is thermal escape, also known as Jeans escape.
The basic idea is that the particles the atmosphere is made of have velocities that follow a Maxwell-Boltzmann distribution. Some small proportion of those particles will have a velocity that exceeds local escape velocity. Above the exobase, the mean free path of those particles tends to exceed the scale height, so there's a good chance that a particle that exceeds local escape velocity will shoot off into space without bumping into anything else on the way.
Important things:
- Low gravity means scale heights are larger, which means the top of the atmosphere is higher, which means that escape velocity at the exobase is lower, which means escape is much easier.
- High temperatures cause the atmosphere to expand, pushing the exobase higher, which means that the escape velocity at the exobase is lower, which means escape is easier.
RMS velocities of molecular gasses tend to be very low, so there's little risk of big molecules shooting off of their own accord. On Earth, what mostly happens is that short-wavelength UV flux at high altitude causes photodissociation which breaks up heavy O2 and O3 into lightweight monatomic radicals which are much faster and therefore much more likely to escape.
- High UV flux at the top of the atmosphere causes photodissociation which produces light, faster monatomic gasses which means that escape is much easier.
So!
Your world orbits a start that has about .33 of the luminosity of the Sun, and it receives about .75 of the stellar irradiance that Earth does. This means it has an orbital radius of ~.66AU. If it has a Bond albedo of ~.2 (brighter than Mercury, darker than Mars, not implausible for a rocky world with little ice on its surface) then it'll have a planetary equilibrium temperature of ~245K... that's not actually that much colder than Earth (which has an equilibrium temperature of 255K).
With half of Earth's gravity but a similar temperature, that's bad news for atmospheric retention.
On the bright side though, a K2V-k3V star with a surface temperature of ~5000K emits substantially less UV than the Sun does... you can use this handy calculator to show that according to Planck's radiation law, UV radiation between 40nm and 400nm makes up only 6.5% of the K-star's output, compared to 11.4% of the Sun's output.
Turning that reduction into actual numbers computing ionization rates and exobase temperatures and Jeans' flux is definitely not something I'm going to do for you, but it is very definitely the case that UV heating of the exosphere and photodissociation of molecular gasses will be much reduced relative to Earth.
So where does that leave you?
This diagram pops up almost often enough that there could be a WB.SE drinking game. Behold, the pretty atmospheric escape chart from wikipedia:
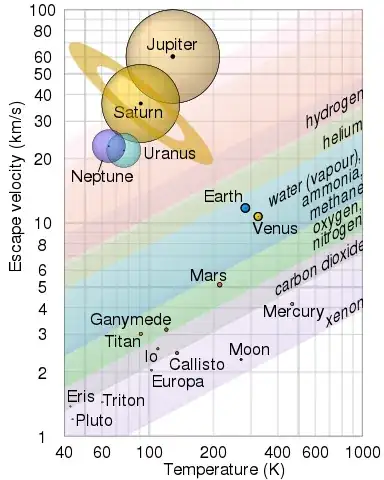
This is a very simplified model (because atmospheric escape is complex) but as a starting point it is nice and easy to describe and work with. The bottom edge of the stripes for the various gasses marks 10 times the root-mean-squared thermal velocity of the relevant gas at the temperature shown on the X axis. (that particular detail isn't in the wikipedia page, but I still haven't got round to telling the person who made the chart)
The 10x threshold is pretty arbitrary, but you can reasonably assume that if your surface escape velocity comfortably exceeds that value then there's a reasonable chance that your atmosphere will hang around for billions of years, rather than merely millions.
As you can see, your world lies above the CO2 line, so it could definitely hang on to a CO2 atmosphere. Although it is within the nitrogen-oxygen region rather than comfortably above it, this chart has been devised for a Sunlike-level of radiation. This means you could reasonably have other gasses too... I'm not going to go ahead and say that you could get a human-breathable atmosphere, but it isn't beyond the realms of possibility.
Do bear in mind that if you wanted oxygen you're probably going to end up with water as well and that means you're going to get a lot of ice, and the surface albedo will shoot up and the equilibrium temperature will plummet. This seems to give you a choice between a freezing snowball world, or a cold dry rocky world. In either case, carbon dioxide and nitrogen both seem like plausible atmospheric constituents, and the atmosphere could be quite thick. If you wanted to handwave in respirators rather than pressure suits, I think you could get away with it.
